The application of controlled levels of negative pressure has been shown to accelerate debridement and promote healing in many different types of wounds. The optimum level of negative pressure appears to be around 125 mmHg below ambient and there is evidence that this is most effective if applied in a cyclical fashion of five minutes on and two minutes off. It is believed that the negative pressure assists with removal of interstitial fluid, decreasing localised oedema and increasing blood flow. This in turn decreases tissue bacterial levels. Additionally, mechanical deformation of cells is thought to result in protein and matrix molecule synthesis, which increases the rate of cell proliferation. Despite the significant costs involved, the technique is said to compare favourably in financial terms with conventional treatments in the management of difficult to heal wounds.
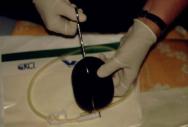
Figure 1 -
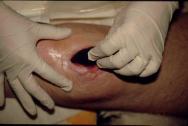
Figure 2 -
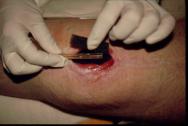
Figure 3 -
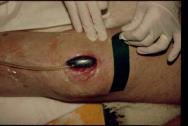
Figure 4 -

Figure 5 -
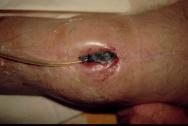
Figure 6 -
Introduction
Vacuum assisted closure (also called vacuum therapy, vacuum sealing or topical negative pressure therapy) is a sophisticated development of a standard surgical procedure, the use of vacuum assisted drainage to remove blood or serous fluid from a wound or operation site.
In essence the technique is very simple. A piece of foam with an open-cell structure is introduced into the wound and a wound drain with lateral perforations is laid on top of it. The entire area is then covered with a transparent adhesive membrane, which is firmly secured to the healthy skin around the wound margin. When the exposed end of the drain tube is connected to a vacuum source, fluid is drawn from the wound through the foam into a reservoir for subsequent disposal.
The plastic membrane prevents the ingress of air and allows a partial vacuum to form within the wound, reducing its volume and facilitating the removal of fluid. The foam ensures that the entire surface area of the wound is uniformly exposed to this negative pressure effect, prevents occlusion of the perforations in the drain by contact with the base or edges of the wound, and eliminates the theoretical possibility of localised areas of high pressure and resultant tissue necrosis.
Development of the vacuum assisted closure technique
The practice of exposing a wound to sub-atmospheric pressure for an extended period to promote debridement and healing was first described by Fleischmann et al in 1993[1], following the successful use of this technique in 15 patients with open fractures. They reported that the treatment resulted in "efficient cleaning and conditioning of the wound, with marked proliferation of granulation tissue". No bone infections occurred in any of the patients although one developed a soft tissue infection, which subsequently resolved with further treatment.
In two further papers, Fleischmann and colleagues described the treatment of 25 patients with compartment syndromes of the lower limb [2] and 313 patients with acute and chronic infections of various types [3]. The average duration of the vacuum therapy treatment for the patients with compartment syndrome was 12.7 (4-31) days with 2.1 (1-8) dressing changes per patient. These wounds were subsequently either closed by secondary suturing (n=20) or by skin grafts following partial closure by suturing (n=5). One patient developed a superficial wound necrosis, which healed spontaneously without invasive surgical treatment.
The average duration of vacuum therapy in the treatment of the 313 patients with infected wounds was 16.7 days with an average of 3.1 dressing changes. Of the 203 wounds with acute infections the majority were subsequently closed by secondary suturing (65.5%) and the remainder by spontaneous epithelialisation (17.2%), skin grafting (12.3%) or flap transfer (2%). Six patients (3%) died. Infection recurred in 3.9% and was managed using another treatment. Unstable scar formations (1%) were treated by free flap transfers.
Further success with topical negative pressure treatment in Germany was reported by Muller [4] following the treatment of 300 patients with infected wounds, and in 1998 Kovacs et al [5] described how 'vacuum sealing' could be used for the treatment of chronic radiation ulcers.
The results of a prospective trial involving 45 patients with soft tissue injuries including sacral pressure ulcers, acute traumatic soft tissue defects and infected soft tissue defects following rigid stabilisation of lower extremity fractures were described by Mullner et al.[6] They reported that in 38/45 patients (84%), the use of the vacuum sealing technique following irrigation and debridement decreased the dimensions of the initial wound, thus facilitating healing time and the eradication of any pre-existing infection.
In the early studies, negative pressure within the wound was achieved by the use of conventional methods such as wall suction apparatus or surgical vacuum bottles. Both these systems are associated with practical problems in terms of the delivery, control and maintenance of the required levels of negative pressure, as discussed by Banwellet al [7].
In 1995, a commercial system for promoting vacuum assisted closure (VAC) was introduced into the United States market. This equipment, called the VAC�, was designed to overcome some of the problems described by Banwell. The heart of the system is a microprocessor-controlled vacuum unit that is capable of providing controlled levels of continuous or intermittent sub-atmospheric pressure ranging from 25 to 200 mmHg.
Two types of unit are available, a mains operated system with a canister volume of 300 ml for patients with limited mobility or very heavily exuding wounds, and a lightweight, battery-powered unit with a canister volume of 50 ml that can delivery therapy to the fully ambulatory patient with minimal to moderate levels of exudate. This system has a battery life of about 17 hours. The large system is fitted with various audible and visual alarms to indicate if the unit is tipped greater than 45 degrees, the canister is full, or the dressing has an air leak.
Vacuum assisted closure: mode of action
In early studies no attempts were made to investigate the physiological basis for the observed clinical effects, or to determine the optimum levels of pressure required. In a seminal paper Morykwas et al [8], addressed both of these issues following a series of animal studies. Deep circular defects, 2.5 cm in diameter, produced on the backs of pigs were dressed with open-cell polyurethane-ether foam with a pore size ranging from 400-600 �m.
In the first series of experiments, a laser Doppler technique was used to measure blood flow in the subcutaneous tissue and muscle surrounding the wounds as these were exposed to increasing levels of negative pressure, applied both continuously and intermittently. Their results indicated that whilst an increase in blood flow equivalent to four times the baseline value occurred with negative pressure values of 125 mmHg, blood flow was inhibited by the application of negative pressures of 400 mmHg and above. A negative pressure value of 125 mmHg was therefore selected for use in subsequent studies.
The rate of granulation tissue production under negative pressure was determined using the same model by measuring the reduction in wound volume over time. Compared with control wounds dressed with saline soaked gauze, significantly increased rates of granulation tissue formation occurred with both continuous (63.3 +/- 26.1%) and intermittent (103% +/- 35.3%) application of negative pressure.
The observation that intermittent or cycled treatment appears more effective than continuous therapy is interesting although the reasons for this are not fully understood. Two possible explanations were advanced by Philbeck et al [9]. They suggested that intermittent cycling results in rhythmic perfusion of the tissue which is maintained because the process of capillary autoregulation is not activated. They also suggested that as cells which are undergoing mitosis must go through a cycle of rest, cellular component production and division, constant stimulation may cause the cells to 'ignore' the stimulus and thus become ineffective. Intermittent stimulation allows the cells time to rest and prepare for the next cycle. For this reason it is suggested that cyclical negative pressure should be used clinically, although some authors [10] [11] suggest that this may follow a 48-hour period of continuous vacuum, which can be applied to exert a rapid initial cleansing effect.
Microbiological studies were also undertaken which involved inoculation of punch biopsy wounds with large numbers of microorganisms. These indicated that, compared with control values, tissue bacterial counts of vacuum-treated wounds decreased significantly after four days [8].
In a final part of the same study using a standard technique, the effect of vacuum therapy was found to increase flap survival by 21% compared with control values [8].
Following these investigations, Morykwas and colleagues postulated that multiple mechanisms might be responsible for these observed effects. In particular, they suggested that removal of interstitial fluid decreases localised oedema and increases blood flow, which in turn decreases tissue bacterial levels. It has since been proposed that the application of sub-atmospheric pressure produces mechanical deformation or stress within the tissue resulting in protein and matrix molecule synthesis [12] and enhanced angiogenesis [13].
Fabian et al [13], using the rabbit ear model, provided further hard evidence for the stimulatory effects of sub-atmospheric pressure on the production of granulation tissue and also demonstrated a trend to enhanced epithelialisation. In experimental partial-thickness burns in pigs, sub-atmospheric pressure was shown to prevent progressive tissue damage in the zone of stasis that surrounds the area of the initial injury. This effect was demonstrable within 12 hours following injury, with treatment times of as little as six hours being sufficient to exert a measurable effect [14]. The authors proposed that removal of oedema fluid containing suspended cellular debris, osmotically active molecules and biochemical mediators, released following the initial injury, may prevent cessation of blood flow.
Clinical experiences with VAC
Following these animal studies, the same research group described the clinical use of the commercial VAC in 300 wounds of varying aetiology [15]. These were treated until completely closed or could be covered with a split-thickness skin graft, or were suitable for surgical reconstruction by rotating a flap on to the healthy granulating wound bed. Overall 296 wounds responded favourably to treatment and the authors concluded that VAC is an extremely efficacious modality for treating chronic and difficult to heal wounds.
Numerous other papers have described the use of VAC in the treatment of a variety of wound types including extensive degloving injuries [16], [17], infected sternotomy wounds [11],[18],[19], and various soft tissue injuries prior to surgical closure [20], grafting or reconstructive surgery [21].
Smith et al [22], in a retrospective review, described the use of VAC over a four-year period in 93 patients who required open abdomen management for a variety of conditions. A total of 171 dressings were applied to the wounds of 38 surgical patients and 55 patients with traumatic injuries. The authors concluded that with careful subsequent management good patient outcomes could be achieved and recommended vacuum assisted closure as the treatment method of choice for open abdomen management and temporary abdominal closure.
Vacuum therapy has also been used in the treatment of donor sites, particularly in areas that are difficult to manage using conventional techniques [23] such as those on the radial forearm [24]. It has been reported that as many as one third of all patients undergoing radial forearm free flaps develop exposed tendon complications and it has been suggested that these individuals may derive particular benefit from the use of VAC therapy [25]. When used as donor site dressings, some authors recommend the use of a low adherent wound contact layer such as Adaptic or paraffin gauze beneath the foam layer [23], [24].
Vacuum assisted closure has also been used in conjunction with split thickness skin grafts in the treatment of burns and is claimed to be particularly useful for body sites with irregular or deep contours such as the perineum, hand or axilla [26], [27]. In all these situations the vacuum helps to hold the graft securely onto the wound bed thus preventing pooling of tissue fluid which would otherwise make the graft unstable.
Molnar et al [28] described how they used VAC in conjunction with skin grafts to treat four patients with full thickness loss of the scalp following a burn injury or excision of an extensive carcinoma. Normally, if such wounds cannot be closed with a flap, the outer surface of the skull is removed to obtain punctate bleeding and a skin graft is applied a week or two later once granulation tissue has started to form. Without this delay the graft take is usually very poor, but with the use of VAC it was possible to apply a successful skin graft immediately after the initial operation.
Numerous case histories describing the successful use of VAC in a variety of non-healing or chronic wounds have also been published. These include a recalcitrant below knee amputation wound and a suspected Brown Recluse Spider bite [29], pressure sores [10], [30], [31], [32], [33], [34], leg ulcers [34], and a group of 30 patients with longstanding wounds that were deemed unsuitable for reconstructive surgery, 26 of whom responded favourably to the treatment [35].
To function correctly, the adhesive membrane applied over the foam wound insert must form an airtight seal with the skin. Obtaining such a seal can be particularly difficult near the anus or vagina or where the surrounding skin is moist. These problems can sometimes be overcome by the use of a hydrocolloid dressing such as Duoderm [15], which is first applied around the wound and used as a base for the adhesive membrane.
Some of the practical problems associated with the application of the VAC system have been discussed previously by Greer et al [33], who developed techniques to allow it to be used successfully on sacral pressure ulcers close to the anus and to multiple large ulcers on the lower extremities.
Fabian et al [13] in a well controlled animal study, investigated the possibility that sub-atmospheric pressure might act synergistically with hyperbaric oxygen (HBO2). They found, however, that although negative pressure increased the rate of healing compared with control values, HBO2 therapy did not offer any significant benefit. The development and use of sub-atmospheric pressures in the management of patients with different types of wounds has been reviewed previously [36].
Cost of treatment
The cost of VAC therapy is not insignificant. In addition to the purchase cost or hire charges of the machine itself, it is necessary to purchase disposable foam dressings and drainage tubes, canisters and adhesive drapes, which together could easily cost in excess of �25 per day. Nevertheless, Philbeck et al [9] claimed that the technique was very cost effective in use. In a retrospective study, they compared the treatment costs of VAC with those of a more conventional therapy by comparing the results of an analysis of the healing rates achieved with the vacuum technique with those recorded for similar wounds in a previously published study. Treatment records of 1032 Medicare patients with 1,170 VAC-treated wounds of all types that had failed to respond to previous interventions were reviewed. From these data, the healing rates of patients nursed on a low air loss surface (LAL) with 43 pressure ulcers (stages III and IV) located on the trochanter and trunk were abstracted and compared with previously published values for a comparable group of patients also nursed on a LAL surface and whose wounds were dressed with saline-gauze packs.
Prior to treatment, the VAC dressed wounds averaged 22.2cm2 compared with 4.3cm2 for the saline-soaked gauze wounds. Wounds dressed with VAC closed at an average of 0.23 cm2 per day compared with 0.090 cm2 for the historical controls. Using these healing rates they calculated that the time to heal a group of patients with wounds 22.2 cm2 in area would be 97 days with VAC and cost $14,546, compared with 247 days with traditional therapy at a cost of $23,465. Whilst acknowledging all the limitations of their study, the authors concluded that negative pressure therapy is an "effective treatment modality for a variety of chronic wounds" producing healing in certain types of pressure ulcers 61% faster than saline soaked gauze whilst reducing costs by 38%. However, further analysis is required comparing treatment costs of VAC with other conventional treatments in the UK.
Method of use
Steps 1-6 demonstrate the technique for vacuum assisted closure:
Step 1
The foam dressing is cut to the approximate size of the wound with scissors (Figure 1) and placed gently into position (Figure 2).

Figure 1 -

Figure 2 -
Step 2
The perforated drain tube is then located on top of the foam and a second piece of foam placed over the top (Figure 3). For shallower wounds, a single piece of foam may be used and the drainage tube is inserted inside it.

Figure 3 -
Step 3
The foam, together with the first few inches of the drainage tube and the surrounding area of healthy skin, is then covered with the adhesive transparent membrane supplied(Figure 4). At this stage it is important to ensure that the membrane forms a good seal both with the skin and the drainage tube.

Figure 4 -
Step 4
The distal end of the drain is connected to the VAC unit, (Figure 5) which is programmed to produce the required level of pressure.

Figure 5 -
Step 5
Once the vacuum is switched on, the air is sucked out of the foam causing it to collapse inwards drawing the edges of the wound in with it (Figure 6).

Figure 6 -
Step 6
Fluid within the wound is taken up by the foam and transported into the disposable container within the main vacuum unit.
The future of vacuum assisted closure
Although numerous papers have been published which suggest the technique may have an important role to play in the management of many types of chronic or infected wounds, the cost of the system is such that some clinicians may be reluctant to use it until further prospective studies have been undertaken to demonstrate its cost effectiveness in routine use.
Even in the absence of such studies, however, few would argue that the technique does not have a role to play in the management of extensive cavity wounds that cannot be surgically closed and are too large to be dressed with conventional dressings. The system may also be of value in the management of heavily exuding wounds, including those with lymphatic involvement.
The principal indications and contraindications to the use of VAC are summarised below (Box 1). In addition, and for obvious reasons, special precautions should be observed when using the technique where haemostasis is difficult, in the presence of active bleeding or in the treatment of patients receiving anticoagulant therapy.
References
1. Fleischmann W, Strecker W, Bombelli M, Kinzl L. [Vacuum sealing as treatment of soft tissue damage in open fractures]. Unfallchirurg 1993; 96(9): 488-92.
2. Fleischmann W, Lang E, Kinzl L. [Vacuum assisted wound closure after dermatofasciotomy of the lower extremity]. Unfallchirurg 1996; 99(4): 283-7.
3. Fleischmann W, Lang E, Russ M. [Treatment of infection by vacuum sealing]. Unfallchirurg 1997; 100(4): 301-4.
4. Muller G. [Vacuum dressing in septic wound treatment]. Langenbecks Arch Chir Suppl Kongressbd 1997; 114: 537-41.
5. Kovacs L, Kloppel M, Geishauser S, Schmiedl S, Biemer E. Vacuum sealing: a new and promising regimen in the therapy of radiation ulcers. Br J Surgery 1998; 85: 70.
6. Mullner T, Mrkonjic L, Kwasny O, Vecsei V. The use of negative pressure to promote the healing of tissue defects: a clinical trial using the vacuum sealing technique. Br J Plast Surg 1997; 50(3): 194-9.
7. Banwell P, Withey S, Holten I. The use of negative pressure to promote healing. Br J Plast Surg 1998; 51(1): 79.
8. Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation.Ann Plast Surg 1997; 38(6): 553-62.